Australia's medical watchdog has issued a warning over an imported weight loss drug that could pose serious health risks if consumed.
Li Da Daidaihua has been found to contain the undeclared substance sibutramine, which is prescription-only in Australia.
The Therapeutic Goods Administration (TGA) said sibutramine became a prescription-only in 2010 due to increased risk of cardiac events and stroke.
READ MORE: 'Incomprehensible evil': Heartbroken families mark first anniversary of Wieambilla shooting
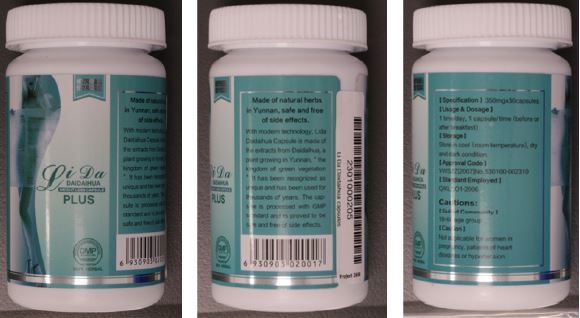
The TGA said the supply of Li Da Daidaihua capsules containing undisclosed sibutramine was illegal.
"Li Da Daidaihua capsules have not been assessed by us for quality, safety or efficacy as required under Australian legislation, and the place of manufacture is not approved by us," the TGA said.
"We advise consumers to exercise extreme caution when purchasing medicines from unknown overseas websites."
The TGA is working with the Australian Border Force to stop future shipments of the product from entering Australia.
Consumers who have purchased the product should stop using it immediately and take the remaining tablets to a pharmacy for safe disposal.
If you suspect you have had a side effect to this product, please report it to the TGA. In addition, if you have concerns about this or other products, you can make a report to us on 1800 020 653 or through our website.
